4 Principle
It is possible to determine the ash of an organic material by three main methods:
a) Direct calcination, i.e. by burning the organic matter and heating the residue at high temperature until
constant mass is reached (method A).
b) Calcination after sulfation, which may be carried out by two different procedures:
⎯ With sulfuric acid treatment after burning, i.e. by burning the organic matter, transforming the
inorganic residue into sulfates with concentrated sulfuric acid and heating the residue at high
temperature until constant mass is reached. This is the common method of obtaining “sulfated ash”
(method B).
⎯ With sulfuric acid treatment before burning, i.e. by heating the organic matter together with
concentrated sulfuric acid up to temperatures where fuming and subsequent burning of the organic
matter occur, and finally heating the residue at high temperature until constant mass is reached
(method C). This procedure may be used if volatile metal halides are liable to evaporate during
burning of the organic matter. It is not applicable to silicones or fluorine-containing polymers.
In each case, the final step of the procedure is calcination at 600 °C, 750 °C, 850 °C or 950 °C until constant mass is reached (see 7.2).
NOTE The mass of the ash may vary with the temperature of ignition. For example, higher temperatures such as 850 °C will convert calcium carbonate and other carbonates to their oxides and thus give lower values for the ash.
5 Reagents (for methods B and C only)
During the analysis, use only reagents of analytical grade and only distilled water or water of equivalent purity.
5.1 Ammonium carbonate, anhydrous.
5.2 Ammonium nitrate, approximately 10 % (by mass) solution.
5.3 Sulfuric acid, ρ = 1,84 g/cm3.
WARNING — Highly corrosive. Handle with suitable skin and eye protection in a fume cupboard.
Reacts exothermically with water.
5.4 Sulfuric acid, 50 % (by volume) solution.
WARNING — Handle with care. Prepare by slowly adding the concentrated acid to water.
6 Apparatus
6.1 Crucible, made of silica, porcelain or platinum, inert to the material tested. The use of a crucible
lid/watch-glass may be beneficial for samples producing a fine particulate ash.
6.2 Gas burner, or other appropriate heat source.
6.3 Muffle furnace or microwave furnace, capable of being maintained at 600 °C ± 25 °C,
750 °C ± 50 °C, 850 °C ± 50 °C or 950 °C ± 50 °C, as appropriate.
6.4 Analytical balance, accurate to 0,1 mg.
6.5 Pipettes, of suitable capacity (for methods B and C only).
6.6 Desiccator, containing an efficient desiccant which does not interact with the ash.
NOTE In certain cases, the ash may have a greater affinity for water than some substances commonly used as desiccants.
6.7 Weighing bottle.
6.8 Fume cupboard.
7 Procedure
7.1 Test portion
Take a quantity of the test sample sufficient to yield 5 mg to 500 mg of ash (see Table 1). In the case of
reinforced materials, take a test portion of 2 g. If the likely quantity of ash is unknown, carry out a preliminary ash determination. Depending on the approximate ash content, choose the size of the test portion to be used from Table 1.
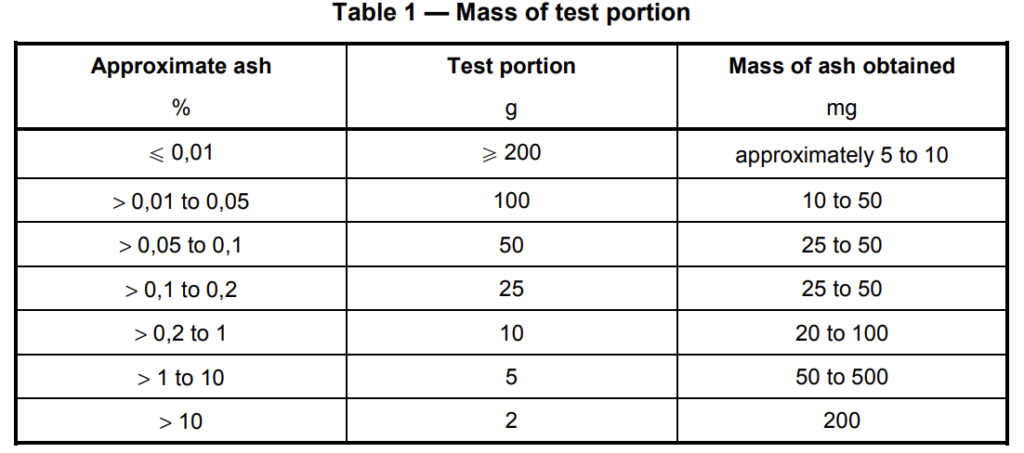
For plastics yielding very low ash, it is necessary to use larger test portions. When it is impossible to burn the whole of the test portion at one time, weigh the required quantity in a suitable weighing bottle and introduce it into the crucible (6.1) in convenient amounts for a succession of burnings until the whole of the test portion has been burnt.
7.2 Test conditions
Calcination shall be continued to constant mass as defined in 7.3.6, but the duration of the calcination in the muffle furnace (6.3) shall not exceed 3 h at the specified temperature.
The choice of the calcination temperature and the use of the sulfation method depend on the nature of the plastic and any additives it may contain. If a choice exists between different satisfactory conditions, choose those that allow the attainment of constant mass in less than 3 h. A higher temperature or the use of sulfation generally shortens the duration of the calcination.
Whichever method — A, B or C — is used, choose one of the following temperature ranges for the final
(calcination) step, unless other temperatures are requested for special technical or commercial reasons:
600 °C ± 25 °C, 750 °C ± 50 °C, 850 °C ± 50 °C, 950 °C ± 50 °C.
Use a fume cupboard for the ashing procedure.
For method A, if it can be conclusively demonstrated for a particular sample type that direct ashing in a muffle furnace without preheating/igniting the sample over a Bunsen flame or equivalent gives the same result, then this version of method A (rapid ashing) is permitted. The use of this rapid ashing method shall be included in the test report.
7.3 Method A — Direct calcination
7.3.1 Prepare the crucible (6.1) by heating it in the muffle furnace (6.3) at the test temperature until constant mass is reached. Allow to cool in the desiccator (6.6) to room temperature for 1 h, or until room temperature is reached, and weigh on the analytical balance (6.4) to the nearest 0,1 mg.
7.3.2 Introduce into the tared weighing bottle (6.7) a test portion (pre-dried as described in the
corresponding material specification) of mass in accordance with Table 1. Weigh again to the nearest 0,1 mg or to 0,1 % of the mass of the test portion. If the test portion corresponding to the amount of ash specified in Table 1 does not more than half fill the crucible, this quantity may be placed directly into the crucible and weighed in it. The procedure described below assumes that this will not be the case, however. High-bulk materials may be compressed into tablets, which may then be broken up into fragments of appropriate size.
7.3.3 Introduce into the crucible enough of the test portion to half fill the crucible. Heat the crucible directly on the burner or other suitable heating device (6.2) to burn slowly until volatile products have been driven off. Repeat the operation until the whole test portion is well charred.
7.3.4 Introduce the crucible into the muffle furnace preheated to the prescribed temperature and calcine for 30 min.
7.3.5 Place the crucible in the desiccator, allow it to cool for 1 h, or until room temperature is reached, and weigh on the analytical balance to the nearest 0,1 mg.
7.3.6 Calcine again under the same conditions until constant mass is reached, i.e. until the results of two
consecutive weighings do not differ from each other by more than 0,5 mg.
7.3.7 If a laboratory can document that their procedure for time and temperature of a single calcination on a given material results in constant mass, then this so-called “rapid method” shall be permitted and the test report shall note a single calcination. In the event of a dispute, the referee method shall be calcination to constant mass.
7.4 Method B — Calcination following sulfuric acid treatment after burning
7.4.1 Proceed as specified in 7.3.1, 7.3.2 and 7.3.3.
7.4.2 After cooling, add sulfuric acid solution (5.4) drop by drop with a pipette of suitable capacity (6.5) to
moisten the residue completely and heat until fuming ceases, avoiding too vigorous boiling.
7.4.3 If traces of carbonaceous materials remain after cooling, add 1 to 5 drops of ammonium nitrate
solution (5.2) and heat until the evolution of white fumes ceases completely.
7.4.4 In order to reconvert metal oxides formed during the preceding steps into sulfates, add, after cooling, about 5 drops of concentrated sulfuric acid (5.3) and heat until there is no further evolution of white fumes, avoiding vigorous boiling or the loss of ash by excessive fuming.
7.4.5 After cooling, add 1 g to 2 g of solid ammonium carbonate (5.1) and heat, avoiding loss of ash, until
the fuming has ceased. Then place the crucible in the muffle furnace preheated to the indicated temperature and proceed as specified in 7.3.4, 7.3.5 and 7.3.6.
7.5 Method C — Calcination following sulfuric acid treatment before burning
7.5.1 This method shall never be used with silicones or fluorine-containing polymers.
7.5.2 Proceed as specified in 7.3.1 and 7.3.2.
7.5.3 Introduce into the crucible enough of the test portion to half fill the crucible. Add with a pipette (6.5) a sufficient amount of concentrated sulfuric acid (5.3) to moisten the material completely. Cover the crucible with a watch-glass. Heat the crucible directly on the burner over a low flame until the organic material begins to decompose.
Continue heating carefully, adjusting the watch-glass so as to allow the acid to be fumed off and making sure that no ash-containing material is lost. With plastics which have a tendency to lose ash-containing material, it is recommended that the crucible with its contents be placed into a holed board made of fireproof material (e.g. ceramic fibre) and heated with a low flame only so that the organic matter smoulders rather than burns. If the initial charge in the crucible was insufficient to yield an acceptable mass of ash, allow the crucible to cool, add more of the test portion and repeat the operations described above until the whole test portion has been burnt. Remove the watch-glass, making sure that no solid particles are adhering to it.
In cases where the sulfuric acid tends to creep over the lip of the crucible or where, despite precautions, some of the test portion tends to be lost by violent reaction (frequently in the case of PVC), the concentrated sulfuric acid may be replaced by a mixture of concentrated acetic and sulfuric acids. The use of these mixed acids shall be agreed between the interested parties and reference made to it in the test report.
7.5.4 Proceed as specified in 7.4.3, 7.4.4 and 7.4.5.
9 Expression of results
The ash or sulfated ash, expressed as a percentage by mass, is given by the formula:

